INTRODUCTION
The in vitro-assisted reproduction techniques have been considered an essential tool for studying oocyte maturation, early embryo development, and animal model research (Prather et al., 2003; Coticchio et al., 2015). It has been demonstrated that the intrinsic high quality of oocyte during maturation is a prerequisite condition for supporting the efficiency of early embryo development as well as fetal growth (Sagirkaya et al., 2007). The communication of oocytes and granulosa cells is a bidirectional process; that is, oocytes affect different functions of granulosa cells around oocyte (Pablo et al., 2018). The oocyte secreted factors regulate the production of steroid hormones by the expression of different genes in granulosa cells, including the genes encoding the LH receptor (Eppig et al., 1997). The first oocyte-specific factor influencing the function of granulosa cells was identified and characterized in mice with targeted deletion of the growth differentiation factor 9 (GDF9) gene, primordial and primary onelayer follicles could be formed, but follicular development beyond the onelayer follicle stage was blocked. And, GDF9 mRNA was synthesized only in oocytes from the primary stage until after ovulation and not in somatic follicular cells in wildtype mouse (Carabatsos et al., 1998; Elvin et al., 1999).The extended expression of GDF9 throughout the oocyte development suggests that GDF9 affects processes in later stages of follicular development (Elvin et al., 2000). The expression of GDF9 have been reported in rodents, goats, sheeps, buffalo, cattle, pig and humans in oocyte, cumulus cells and ganulosa cells and there is convincing evidence that they are important for ovarian function (Prochzka et al., 2004; Paradis et al., 2008; Hosoe et al., 2009; Li et al., 2014; Mester et al., 2015; Pan et al., 2015; Chang et al., 2016; Haas et al., 2016; Mishara et al., 2016; Silva et al., 2016; Kona et al., 2018; Pablo et al., 2018). In current study, we aimed to evaluate the expression of GDF9 at the mRNA level in porcine ovarian tissue, COC, CC and DO at different stages of maturation in vitro.

2. MATERIAL AND METHODS
2.1. Material, chemicals and supplies
Pig ovaries were collected from a local slaughter house. All chemicals and reagents were purchased from Sigma-Aldrich (Oakville, ON, Canada), unless otherwise stated. The experiments were carried out at the Animal Embryo Technology Lab, Research Institute for Biotechnology and Environment and Faculty of Biological Sciences, Nong Lam University in Ho Chi Minh City from Nov, 2021 to May, 2022. 2.2. Methods 2.2.1. Ovary and Oocyte collection Collection of ovaries and oocyte aspiration were carried out as described by Nguyen et al. (2012). Briefly, porcine ovaries collected from a local abattoir and transported to the laboratory at approximately 30-35°C within 2h post collection. The cumulus-oocyte complexes (COCs) were manually aspirated from follicles 3-7mm in diameter using an 18- ga needle attached to a 10-ml syringe (Nguyen et al., 2012). Cumulus-oocyte complexes were searched under a stereomicroscope and washed (three times) in wash medium. All COCs with more than two layers of cumulus cells and uniform cytoplasm were selected to culture for further use.
2.2. In vitro maturation Cumulus-oocyte complexes were washed (three times) in maturation media containing TCM-199 containing Earl’s salts, L-glutamine and Sodium bicarbonate (Sigma M4530, USA), supplemented with 10% follicular fluid, 0.8% BSA (Bovine serum albumin), 100 IU/ mL Penicillin G sodium salt and 100 IU/mL Streptomycin sulfate salt. Add 10 IU/mL of hCG hormone (human Chorionic Gonadotropin for the first 22h of culture (TCM+ ) and no hormone for 22h of culture after (TCM- ). Groups of 20 COCs were placed in 100 µl droplets of maturation media under mineral oil and incubated for 44h at 39°C, 5% CO2 in air.
2.3. Detection of GDF9 mRNA by Reverse Transcription-Polymerase Chain Reaction Extraction of mRNA: Total RNA was extracted from samples of ovarian tissue derived from (A) small follicle (SF), (B) medium follicles (MF) and (C) large follicles (LF) or COCs at the different time points of maturation: (D) 0h, (E) 22h and (F) 44h poat culture or (G): cumulus cell (CC) or (H) denuded oocyte (DO) as showed in Figure 1. The extraction mRNA following the TRIsureChloroform (Méndez et al., 2011). Primer design: Taking into account the sequences recently published by the National Center for Biotechnology Information (NCBI) and the data base of the porcine genome sequence, specific primers were designed in order to amplify porcine gene coding regions GDF9 using the online software PrimerBLAST. Additionally, specific primers GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were also used as an internal control. The primer sequence for GDF9 (NM_001001909.1) with F 5’-GGGTGTACCATCCTCTCGTC-3’ and R 5’- TGTGAACCGGAGAGCCATAC-3’; for GAPDH (AF017079) with F 5’-AGCAATGCCTCCTGTACCAC-3’ and R 5’-AAGCAGGGATGATGTTCTGG-3’. These primers were expected to generate a 269 and 187bp cDNA fragment for GDF9 and GAPDH, respectively.
Ampplification by the reverse transcriptionpolymerase chain reaction (RT-PCR): The RTPCR was carried out by MyTaq One-Step RT-PCR Kit (Bioline). For each sample, the amplification of each gene was run in a separate tube. The reaction conditions were as follows: cDNA synthesis at 45o C for 20 min, predenaturation at 95o C for 1 min, and then 35 numbers of PCR cycles consisting of denaturation (95o C for 10 sec), annealing (59o C for 20 sec), extension (72o C for 30 sec), and final extension (72o C for 7 min). For semiquantitative RT-PCR: Products of the RT-PCR were separated by electrophoresis on 1.5% agarose gel and visualized by Gelred. The intensity of the objective bands was determined by scanning densitometry using Image J Version 1.29 free software (National Institute of Mental Health, Bethesda, MD). The relative abundance of GDF9 mRNA was expressed as the ratio of GDF9 to GAPDH.
2.4. Contents
2.4.1. Determination of GDF9 expression at the mRNA level in ovarian tissue Ovarian tissue from SF, MF and SF (Figure 1A, 1B, 1C) was collected separately for extraction of mRNA.
2.4.2. Determination of GDF9 expression at the mRNA level in COCs at different stages of matuarion in vitro COCs were cultured in 39o C, 5% CO2 in air and COCs were collected at 0, 22 and 44 h post culture as showed in the Figure 1D, 1E, 1F and subjected to extract mRNA.
2.4.3. Determination of GDF9 expression at the mRNA level in CC and DO at 0h post culture Right after collection of COCs, the COCs were removed cumulus cell to collect denuded oocyte without cumulus cells (DO) and cumulus cells (Figure 1G and H) then subjected to extract mRNA.
2.5. Data analysis All data were subjected into one way ANOVA analysis, followed by Tukey’s test using Minitab 18.1 software. The data are presented as Mean±SEM at least from three
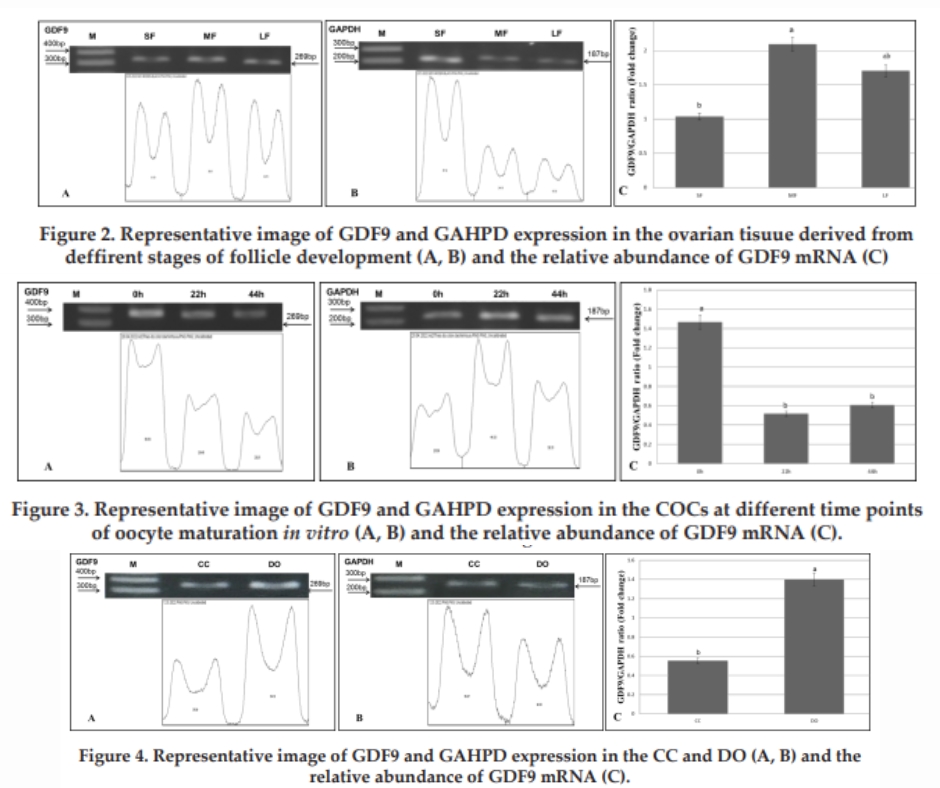
Data from Figure 2 showed that the mRNA of GDF9 was detected in ovarian tissue derived from SF, MF and LF (Figure 2A). GAPDH gene was also applified in all kinds of ovarian tissued (Figure 2B). The relative abundance of GDF9 mRNA (Figure 2C) was lowest (1.03±0.26) in ovarian tissue derived from SF, highest (2.09±0.18) in MF significant difference compared to SF (P<0.05) then declined in ovarian tissue derived LF (1.70±0.43). No significant difference between SF and LF or LF and MF was found (P>0.05). In sheep, Pan et al. (2018) documented that the GDF9 mRNA levels in the ovary were significantly higher (P<0.01) than in other tissues, with lower levels being found in the pituitary, liver, hypothalamus, spleen, cerebellum, uterus, lung, oviduct, and heart. In current study, it is the first report on expreesion of GDF9 at the mRNA level in porcine ovarian tissue derived from different stages of follicle development.
3. RERULTS AND DISCUSSION
3.1. Determination of GDF9 expression at the mRNA level in ovarian tissue derived from small folliles (SF), medium follicles (MF) and large follicles (LF) After electrophoresis of RT-PCR product of target gene using mRNA extracted from avarian tissue samples, the relative abundance of mRNA in GDF9, GAPDH bands was measured and presented in Figure 2.
3.2. Determination of GDF9 expression at the mRNA level in COCs at different stages of matuaration in vitro Using extracted RNA from COCs collected at 0, 22 and 44h of maturation culture conducted RT-PCR, after electrophoresis of RT-PCR product, the relative abundance of mRNA in GDF9, GAPDH bands was measured and presented in Figure 3. Results from Figure 3 indicated that the mRNA of GDF9 was detected in COCs at 0, 22 and 44h post culture in vitro (Figure 3A). GAPDH gene was also applified in all treatments (Figure 3B). The relative abundance of GDF9 mRNA (Figure 3C) was highest (1.46±0.36) in COCs collected at 0h of maturation culture and significant difference compared to grouped COCs at 22h (0.51±0.10) and 44h (0.60±0.10). Significant differences were found in COC obtained at 0h compared with COC obtained at 22 and 44h post culture replicates.
in vitro (P<0.05). Several studies documented that expression of mRNA GDF9 was highly expressed in the COCs at the beginning of maturation culture and slowly declined throughout the process (Lee et al., 2008; Li et al., 2008). The level of mRNA GDF9 in GVBD and MI stages of nuclear maturation was significantly higher than GV stage and then declined to MII stage of porcine oocyte (Lin et al., 2013) and human oocytes maturation in vitro (Zhao et al., 2010)
3.3. Determination of GDF9 expression at the mRNA level in CC and DO at 0h post culture Taken the results from Figure 3, using extracted RNA from CCs and DOs collected at 0h of maturation culture performed RTPCR, after electrophoresis of RT-PCR product, the relative abundance of mRNA in GDF9, GAPDH bands was measured and presented in Figure 4.
Results from Figure 4 showed that the mRNA of GDF9 was detected in CC and DO at 0h post culture in vitro (Figure 4A). GAPDH gene was also applified in all treatments (Figure 4B). The relative abundance of GDF9 mRNA (Figure 4C) in the DO was higher than CC group (1.39±0.40 and 0.55±0.26, respectively, P<0.05). Lee et al. (2008) reported that the GDF9 mRNA was strongly expressed in the oocyte as compared to those in cumulus and granulosa cells of female domestic animal. In pig, Prochazka et al. (2004) also documented the expression of mRNA of GDF9 gene was dominant expressed in COC as compared to CC, it is the same trend with our current study.
4. CONCLUSION The expression of GDF9 gene is in relation to oocyte maturation in the manner and can be considered as a candidate marker gene for oocyte quality evaluation in vitro
REFERENCES
1. Carabatsos M.J., Elvin J., Matzuk M.M. and Albertini D.F. (1998). Characterization of oocyte and follicle development in growth differentiation factor-9- deficient mice. Dev. Biol., 204: 373-84. 2. Chang H.M., Qiao J. and Leung P.C.K. (2016). Oocyte– somatic cell interactions in the human ovary—novel role of bone morphogenetic proteins and growth differentiation factors. Hum. Rep. Update, 23(1): 1-18. 3. Coticchio G., Dal Canto M., Mignini Renzini M., Guglielmo M.C., Brambillasca F., Turchi D., Novara P.V. and Fadini R. (2015). Oocyte maturation: Gametesomatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Rep. Update, 21: 427-54. 4. Elvin J.A., Changning Y. and Matzuk M.M. (2000). Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Pro. Nat. Aca. Sci. USA; 97: 10288-93. 5. Elvin J.A., Clark A.T., Wang P. and Wolfman N.M. (1999). Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol. Endocrinol., 13: 1035-48. 6. Eppig J.J., Wigglesworth K., Pendola F., and Hirao Y. (1997). Murine oocytes suppress expression of luteinizing hormone-receptor messenger ribonucleic acid by granulosa cells. Biol. Rep., 56: 976-84. 7. Haas C.S., Rovani M.T., Oliveira F.C., Vieira A.D., Bordignon V., Gonçalves P.B.D., Ferreira R. and Gasperin B.G. (2016). Expression of growth and differentiation Factor 9 and cognate receptors during final follicular growth in cattle. Anim Reprod; 13(4): 756-61. 8. Hosoe M., Kaneyama K., Ushizawa K., Hayashi K. and Takahashi T. (2011). Quantitative analysis of bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9) gene expression in calf and adult bovine ovaries. Rep. Biol. Endocrinol., 9(1): 33. 9. Hou-Kuan L., Ting-Yung K., Hsiu-Shan Y., Lih-Ren C., S. Shoei-Lung L. and Hurng-Wern H. (2008). Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos, 103(3-4): 1-22. 10. Kona S.S.R., Praveen C.V., Siva Kumar A.V.N., Srividya D., Padmaja K. and Rao V.H. (2016). Quantitative expressionpatterns of GDF9 and BMP15 genes in sheep ovarian follicles grown in vitro or cultured in vitro. Theriogenology; 85(2): 315-22. 11. Lee G.S., Kim H.S., Hwang W.S. and Hyun S.H. (2008). Characterization of porcine growth differentiation factor-9 and its expression in oocyte maturation. Mol. Rep. Dev., 75(5): 707-14. 12. Li Y., Li R.Q., Ou S.B., Zhang N.F., Ren L., Wei L.N., Zhang Q.X. and Yang D.Z. (2014). Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Rep. Biol. Endocrinol., 12(1): 81. 13. Lin Z.L., Li Y.H., Xu Y.N., Wang Q.L., Namgoong S., Cui X.S. and Kim N.H. (2013). Effects of Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15 on thein vitroMaturation of Porcine Oocytes. Rep. Dom. Anim., 49(2): 219-27. 14. Mester B., Ritter L.J., Pitman J.L., Bibby A.H., Gilchrist R.B., McNatty K.P., Juengel J.L. and Mclntosh C.J. (2015). Oocyte expression, secretion and somatic cell interaction of mouse bone morphogenetic protein 15 during the peri-ovulatory period. Rep. Fertil Dev., 27(5): 801-11. 15. Mishra S.R., Thakur N., Somal A., Parmar M.S., Reshma R., Rajesh G, Yadav V.P., Bharti M.K., Bharati J., Paul A., Chouhan V.S., Sharma G.T., Singh G. and Sarkar M. (2016). Expression and localization of fibroblast growth factor (FGF) family in buffalo ovarian follicle during different stages of development and modulatory role of FGF2 on steroidogenesis and survival of cultured buffalo granulosa cells. Res. Vet. Sci., 108: 98-11. 16. Méndez V., Avelar E., Morales A., Cervantes M., Araiza A. and D. González (2011). A rapid protocol for purification of total RNA for tissues collected from pigs at a slaughterhouse. Genet. Mol. Res., 10: 3251-55. 17. Nguyen N.T., Lo N.W., Chuang S.P., Jian Y.L. and Ju J.C. (2012). Sonic hedgehog supplementation of oocyte and embryo culture media enhances development of IVF porcine embryos. Rep., 142: 87-97. 18. Pablo R.S., S. Coria M., Barrionuevo M.G., Hernández O., Callejas S. and Palma G.A. (2018). Gene expression of growth factor BMP15, GDF9, FGF2 and their receptors in bovine follicular cells Revista MVZ Córdoba, 23(3): 6778-85. 19. Pan ZY, Di R, Tang QQ, Jin HH, Chu MX, Huang DW, He J.N., Liu Q.Y., Hu W.P., Wang X.Y., Yao Y.X., Liu L. and Song C.L. (2015). Tissue-specific mRNA expression profles of GDF9, BMP15, and BMPR1B genes in prolific and non-prolific goat breeds. Czech J. Anim. Sci., 60(10): 452-58. 20. Pan Z.Y., Wang X.Y., Di R., Liu Q.Y., Hu W.P., Cao X.H., Guo X.F., He X.Y., Lv S.J., Li F.K., Wang H. and Chu M.X. (2018). A 5-Methylcytosine site of growth differentiation factor 9 (GDF9) gene affects its tissuespecific expression in sheep. Animals, 8: 200. 21. Paradis F., Novak S., Murdoch G.K., Dyck M.K., Dixon W.T. and Foxcro G.R. (2009). Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFβ-R1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Rep., 138(1): 115-29. 22. Prather R.S., Hawley R.J., Carter D.B., Lai L. and Greenstein J.L. (2003). Transgenic swine for biomedicine and agriculture. Theriogenology, 59: 115- 23. 23. Prochazka R., Nemcova L., Nagyova E. and Kanka J. (2004). Expression of Growth Differentiation Factor 9 messenger RNA in porcine growing and preovulatory ovarian follicles. Biol. Rep., 71(4): 1290-95. 24. Sagirkaya H., Misirlioglu M., Kaya A., First N.L., Parrish J.J. and Memili E. (2007). Developmental potential of bovine oocytes cultured in different maturation and culture conditions. Anim. Rep. Sci., 101: 225-40. 25. Shimizu T., Miyahayashi Y., Yokoo M., Hoshino Y., Sasada H. and Sato E. (2004). Molecular cloning of porcine growth differentiation factor 9 (GDF-9) cDNA and its role in early folliculogenesis: direct ovarian injection of GDF-9 gene fragments promotes early folliculogenesis. Rep., 128(5): 537-43. 26. Silva J.R.V., van den Hurk R. and Figueiredo J.R. (2016). Ovarian follicle development In vitro and oocyte competence: advances and challenges for farm animals. Dom. Anim. Endocrinol., 55: 123-35. 27. Zhao S.Y., Qiao J., Chen Y.J., Liu P., Li J. and Yan J. (2010). Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril, 94: 261-67.