INTRODUCTION
Genotype-based breeding narrows the generation gap by pre-selecting traits when animals are young since genes allow trait testing regardless of sex or age, increasing accuracy when selecting difficult traits, and reducing test populations or offspring via immediate genotype selection. To achieve targeted genetic improvement in livestock, the genes controlling desirable and undesirable traits must be characterized, which is not yet complete. Marker-assisted selection (MAS) in breeding helps to achieve this goal, especially in cases where pedigree data are unavailable and the targeted traits have low heritability (Dentine, 1999). Identifying genes that control specific traits can be approached in several ways. Traits controlled by a single gene can be determined by observing variations through studying physiology and the biochemical pathways involved. For traits that are controlled by multiple genes, genetic mapping can be used. Maps with genes controlling specific traits are generated using markers of associated chromosomal regions. Identifying genes that regulate traits by genetic mapping has been done to locate the quantitative trait loci on chromosomes and identify genes in that region. Although not expected to significantly improve traits with high heritability, MAS is believed to be valuable in improving traits with low heritability such as carcass and reproduction traits.

SOME COMMON MOLECULAR MARKERS
Microsatellites
Microsatellites are tandemly repeated tracts of DNA composed of 1-6 base pair (bp) long units. Microsatellites loci are also known as simple sequence repeats (SSR), short tandem repeats (STR), simple sequence tandem repeats (SSTR), and variable number tandem repeats (VNTR), simple sequence length polymorphisms (SSLP). They are ubiquitous in prokaryotes and eukaryotes, present even in the smallest bacterial genomes (Field and Wills, 1996). Microsatellites can be found anywhere in the genome, both in coding and non-coding regions. Because of their high mutability, microsatellites are thought to play a significant role in genome evolution by creating and maintaining quantitative genetic variation. In promoter regions, the length of SSRs may influence transcriptional activity (Kashi et al., 1997). Hancock (1995) indicated that SSRs in exons are less abundant than in non-coding regions and that different taxa exhibit different preferences for SSR types (Tautz and Schlötterer, 1994).
The SSR approach offers multiple advantages, including several alleles in a locus, even distribution throughout the genome, co-dominant direction, high polymorphism and specificity, experiment reproducibility, minimal DNA usage, low cost, and ease of implementation. However, microsatellites also have the following disadvantages: high initial development expenses, lengthy development times, and high development costs. Mutations in the primer annealing sites may cause misclassification of heterozygotes as homozygotes when null alleles are present. Microsatellite markers aid in the identification of neutral biodiversity but provide no information regarding the functional characteristics of biodiversity (Teneva, 2009). Currently, SSR is the indicator of choice for forensic record studies, population genetics, and wildlife studies.
Restriction fragment length polymorphism
The RFLP marker, developed by Botstein et al. (1980), uses bacterial restriction enzymes to cut DNA molecules at specific recognition sequences, typically 4-6bp long. Many restriction enzymes have unique recognition sites. Variations in restriction enzyme recognition sites were discovered by cleaving genomic DNA with a restriction enzyme and electrophoresizing the fragments. Numerous segments were created to test simultaneously because of the genome’s recognition sites. The RFLP marker has a high degree of polymorphism, is co-dominant, can distinguish heterozygous and homozygous individuals, and is highly repeatable. This technique is commonly used for the detection and identification of gene polymorphisms and for recombinant DNA technology in livestock (Beuzen et al., 2000). The PCR-RFLP technique is performed by restricting the PCR product of specific loci with one or more restriction enzymes and separating the cut DNA fragments on agarose or polyacrylamide gels. Primer pairs are designed based on the sequence information of the DNA or cDNA on the Genbank. These are co-dominant markers and are specific loci used to distinguish homozygotes from heterozygotes (Konieczny and Ausubel, 1993). PCR-RFLP is commonly used in diagnostic testing to determine the genotype in a known genetic mutation.
Single nucleotide polymorphisms
A single nucleotide polymorphism (SNP) is a variation in the DNA sequence that occurs at a single nucleotide in the genome (Scherf and Pilling, 2015). SNP expression sites in the genome are places where DNA sequences are distinguished by a single base when two or more individuals are compared. This difference in nucleotides can lead to changes in particular traits or phenotypes. SNPs are by far the most common form of sequence change in the genome. Several reasons are responsible for the growing emphasis on employing SNPs as genetic analysis markers. First, they are more prevalent than other forms of polymorphisms and provide more possible markers near or at any area of interest. For example, human genomic DNA contains an SNP greater than 1,000bp (Landegren et al., 1998). Secondly, some SNPs are positioned in the coding area and influence the protein’s function directly. These SNPs are directly responsible for some interindividual variances in economically significant features. In addition, SNPs are more stable than microsatellites and more dependable for genetic study than microsatellites (Lipshutz et al., 1999). Among the molecular markers, SNPs are promising for association studies. These markers are abundant and exhibit a low mutation rate, which facilitates genotyping (Chen and Sullivan, 2003). Many studies have been performed on the association between SNPs in candidate genes and metabolic pathways in several species. For example, Wong et al. (2004) identified 2.8 million SNPs in the chicken genome. This abundance of available SNPs may aid in the future mapping of causative polymorphisms underlying complex traits in chickens. In addition, stability and abundance made SNP popular and interesting. SNP is a co-dominant indicator; therefore, it may assess individual genotypes in a population.
Insertion/Deletion
The Insert/Delete polymorphism, abbreviated In/Del, is a type of genetic variation in which a nucleotide can be inserted or deleted in the nucleotide sequence of a gene. Although not as common as SNPs, In/Dels are common in the genome. In/Del includes a total of 3 million of the 15 million known genetic variations (1,000 Genomes Project Consortium, 2010). An In/Del in the coding region of the gene that is not a multiple of 3 nucleotides leads to a frameshift mutation. Shifting reading frames and transcriptional sequences of genes can code for a different amino acid or lead to premature codon inactivation, altering protein structure and function. An In/Del can change the DNA sequence and shift the framework, thereby altering the sequence of amino acids produced, resulting in abnormal protein production even if no protein is produced.
The In/Del mutation is a source of genetic variation, often segregated into short and long In/Del due to different approaches to longer variants. One short In/Del (less than 50bp) for 8 human SNPs (Wilson et al., 2001), represents a ratio of variation. In/Del is believed to contribute more to sequence diversity than SNPs, regarding the number of distinct bases. In addition, it has been suggested that a short In/Del could be instrumental in maintaining an optimal intron size.
APPLICATION OF GENETIC MARKERS FOR LIVESTOCK IN VIETNAM
Biodiversity and conservation of animal genetic resources
In cattle, Pham Doan Lan et al. (2008) assessed the genetic diversity and genetic structure of the cattle population in Ha Giang province. A total of 23 microsatellites were used to the genotype of 530 cattle individuals. The results showed that 205 alleles were observed in the total population. The allelic diversity (average number of alleles per locus) was 8.9±2.05. The average observed and expected heterozygosity for the population was 0.67 and 0.73, respectively. The polymorphic information content (PIC) value of each locus ranged from 0.50 to 0.84. The average genetic variation between district cattle populations was very low with an FST value of 0.013. In chickens, Le Thi Thuy et al. (2009) used 20 pairs of microsatellite primers to study the genetic diversity of 5 local chicken breeds in Vietnam (Ac, Choi, H’Mong, Ho, and Tre chicken). The results showed that the populations of these breeds were highly diverse and the degree of genetic dispersion among chicken populations was very large. These five chicken breeds were divided into 3 groups including (1) Tre chicken, (2) Ac chicken, and (3) others, in which the genetic distance of Choi and Ho chicken was closest. In addition, Ngo Thi Kim Cuc and Nguyen Van Ba (2019) assessed genetic diversity and genetic differentiation between Lac Son chicken and other native chicken breeds using 20 microsatellite markers. The results indicated that microsatellite markers studied were polymorphic with an average of 6.73 alleles/locus. The genetic diversity of Lac Son chicken was on the average level with the expected heterozygosity of 0.60 and the number of alleles/locus was 6.05. The inbreeding coefficient of Lac Son chicken was very low. The lowest genetic distance was found between Lac Son chicken and Dong Tao chicken but the highest genetic distance was found between Lac Son chicken and Tai Do chicken. Sub-structuring of the Vietnamese chicken breeds was related to their geographical distribution distance. Ri, Dong Tao, and Mia chicken in the Red River Delta were in the same group, while Lac Son chicken in the northern central region was separated and different from the Tai Do chicken (Red Jungle Fowl) breed.
Identification of candidate genes associated with economic traits
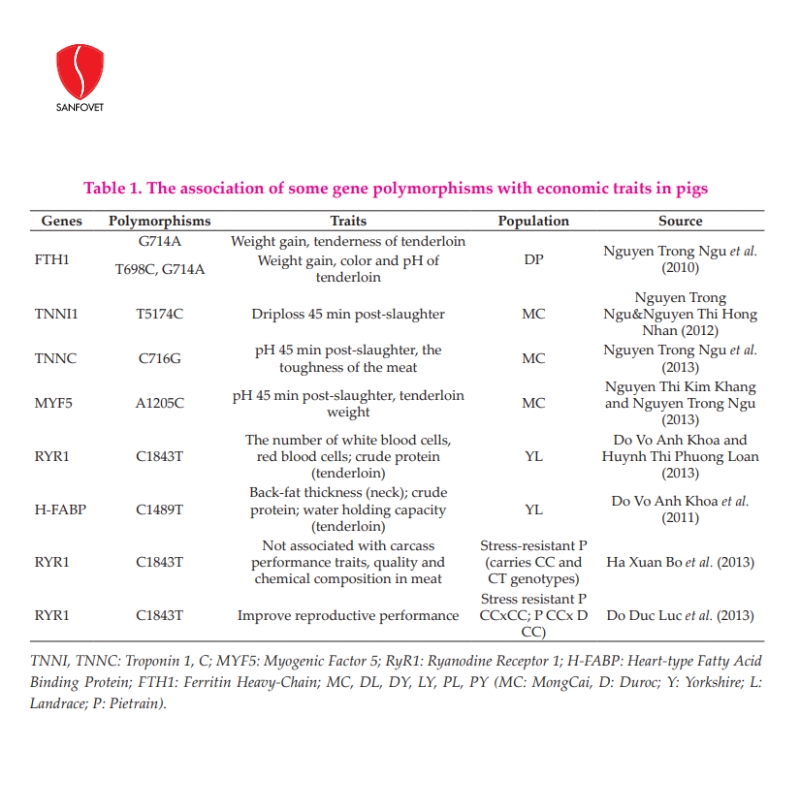
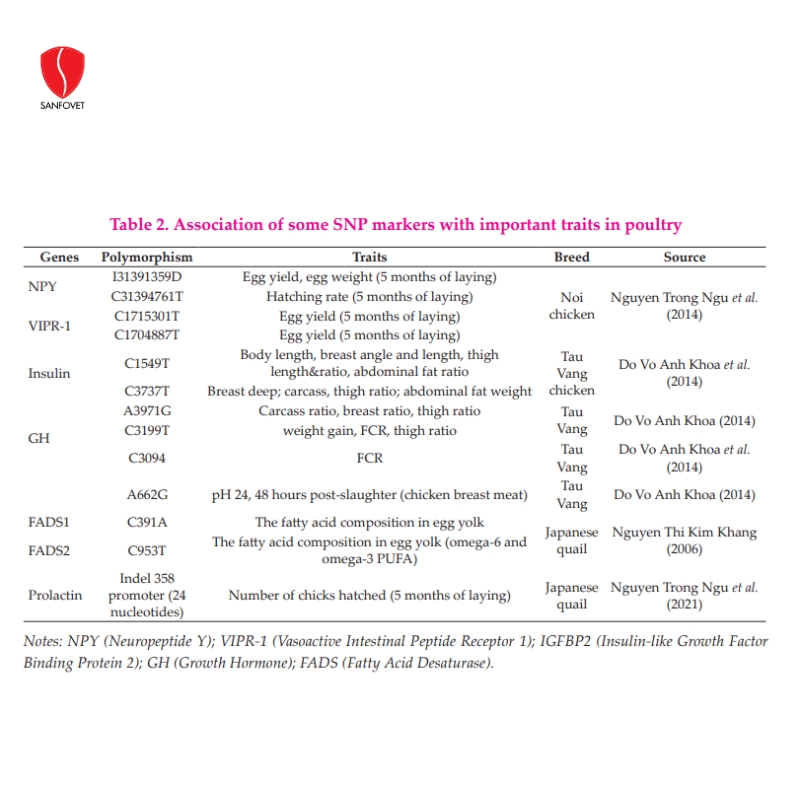
In pigs, several genes are associated with weight gains such as HAL, GH, and LEP. Nguyen Ngoc Tuan and Tran Thi Dan (2005) reported that pigs carrying the HAL gene with genotype “nn” had a low growth rate but higher back fat thickness than “NN” or “Nn” pigs. For imported pig breeds, carcass ratio, carcass length, back-fat thickness, lean percentage, and fat percentage did not differ between genotypes “NN” and “Nn” but the tenderloin area group of pigs “Nn” was larger than pigs “NN” with significance (P<0.05). The group of pigs with the gene “nn” adversely affected reproductive parameters, while the group of pigs “NN” had the highest number of newborns per litter. Nguyen Huu Tinh et al. (2020a) selected the terminal sire pig line TS3 (Duroc) performing with high production based on testing the genotype of H-FABP, MC4R, and PIT-1, and the evaluation of breeding values estimated by the BLUP procedure and genotype. In the 3rd generation, TS3 pigs were improved remarkably for tested performance traits as compared to the original generation, such as 932g for ADG, 10.8mm for BF, 63.8mm for loin depth, 144.9 days for age to 100kg, 2.45 for FCR, 62.1% for lean meat and 3.22% for intramuscular fat. Commercial crossbred pigs used TS3 as terminal sires with 921 g for ADG, 2.39 for FCR, and 61.4% for lean meat. In addition, Nguyen Huu Tinh et al. (2020b) selected 2 pig lines SS1 (Landrace) and SS2 (Yorkshire), and parental crossbred sows (SS12 and SS21) performing with high reproduction based on identifying FSHB and PRLR genotypes and evaluation of breeding values were estimated by BLUP procedure. Other markers associated with traits of interest in pigs are shown in Table 1. In chickens, Tran Xuan Hoan et al. (2000, 2001) analyzed GH gene polymorphisms in local chicken breeds and concluded that there was no association between genotypes and egg production at 36 weeks of age and body weight at 20 and 36 weeks old. Studying the influence of PIT1 gene polymorphism on performance traits of the Tau Vang chicken breed, Le Thi Thu Ha et al. (2015) identified 3 genotypes AA, AB, and BB in the flock of Tau Vang. The authors found only the effect of this polymorphism on the egg weight of chickens, in which hens carrying genotype AA gave the highest egg weight. Recently, Tran Thi Binh Nguyen et al. (2020) investigated SNP of candidate genes in Lien Minh chickens with the allele frequencies obtained were 0.97 and 0.03 for alleles A and G (GHi3), respectively; in IGFBP2, 0.47 for the A allele and 0.53 for G; in PIT1, 100% for allele B. These polymorphic loci (GHi3, PIT1, and IGFBP2) followed the Hardy-Weinberg equilibrium in the Lien Minh chicken population. These were the initial results, which could be used to analyze the association of molecular markers and grow traits in Lien Minh chickens. Other studies related to growth traits in broilers, egg producing traits in laying hens, and egg quality characteristics in quails are presented in Table 2.
CONCLUSIONS
Practically, molecular markers are applicable to a variety of fields and animal species. Within the scope of this paper, only two topics are addressed: genetic diversity and candidate genes in the primary target species of pigs, chickens, and quails. In addition to the aforementioned topics, molecular markers have been researched in dairy cows to enhance milk yield and quality. In addition, recent research on potential genes associated with disease resistance has been discussed. When it comes to livestock production in Vietnam, the use of molecular markers in breeding is important, particularly for local breeds, in order to increase productivity while maintaining the product quality that satisfies consumer demand.
REFERENCES
1. Beuzen N.D., Stear M.J. and Chang K.C. (2000). Molecular markers and their use in animal breeding. Vet. J., 160(1): 42-52. 2. Botstein D., White R.L., Skolnick M. and Davis R.W. (1980). Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet., 32: 314-31. 3. Brookes A.J. (1999). The essence of SNPs. Genetics, 234(2): 177-86. 4. Chen X. and Sullivan P.F. (2003). Single nucleotide polymorphism genotyping: biochemistry, protocol, cost and throughput. Pharmacogenomics J., 3(2): 77-96. 5. Dentine M.R. (1999). Marker-assisted selection. In The genetics of cattle (Fries R. and Ruvinsky A., eds). CABI Publishing, New York, 497-10. 6. Do Vo Anh Khoa and Huynh Thi Phuong Loan (2013). Skeletal ryanodine receptor gene associated with chemical compositions of meat and physiochemical parameters of blood. Biotechnol. in Anim. Husb., 29(3): 431-40. 7. Do Duc Luc, Ha Xuan Bo, Nguyen Chi Thanh, Nguyen Xuan Trach and Vu Dinh Ton (2013). Reproductive performance of othe nucleus herd of stress negative Piétrain and Duroc swine raised at the animal farm of Hanoi Uni of Agriculture. J. Sci. Dev., 11(1): 30-35. 8. Do Vo Anh Khoa (2012). Effects of C1032T single nucletotide popymorphim of the IGFBP2 gene on meat yield traits in Tau Vang chicken. Can Tho Uni. J. Sci., 24b: 1-7. 9. Do Vo Anh Khoa (2014). Genetic polymorphism of the C3199T GH gene associated with performance traits of Tau Vang chicken meat. J. Anim. Sci. Technol., 51: 70-78. 10. Do Vo Anh Khoa, Nguyen Huy Tuong and Nguyen Thi Dieu Thuy (2011). Effect of H-FABP genotype on blood physiological and biochemical traits, performance and quality of pork. J. Sci. Dev., 4: 673-82. 11. Do Vo Anh Khoa, Nguyen Thị Kim Khang, Nguyen Minh Thong and Le Thi Men (2014). The polymorphism of the T3737C insulin gene associated with some carcass traits of the Tau Vang chicken. JAHST, 4: 12-19. 12. Field D. and Wills C. (1996). Long polymorphic microsatellites in simple organisms. Pro. R. Soc. Lond, 263: 209-15. 13. Hancock J.M. (1995). The contribution of slippage-like processes to genome evolution. J. Mol. Evol., 41: 1038- 47. 14. Ha Xuan Bo, Do Duc Luc and Dang Vu Binh (2013). Effect of Halothane Genotype, Gender on Carcass Characteristics and Meat Quality of Stress Negative Piétrain Pigs. J. Sci. Dev., 11(8): 1126-33. 15. Kashi Y., King D. and Soller M. (1997). Simple sequence repeats as a source of quantitative genetic variation. Trends Genet., 13: 74-78. 16. Konieczny A. and Ausubel F.M. (1993). Procedure for mapping Arabidopsis mutations using co-dominant ecotype specific PCR-based markers. Plant. J., 4: 403- 410. 17. Landegren U., Nilsson M. and Kwok P.Y. (1998). Reading bits of genetic information: methods for single nucleotide polymorphism analysis. Genome Res., 8(8): 769-76. 18. Lipshutz R.J., Fodor S.P., Gingeras T.R. and Lockhart D.J. (1999). High density synthetic oligonucleotide arrays. Nature Genet., 21(1): 20-24. 19. Le Thi Thuy, Nguyen Trong Binh and Nguyen Van Ba (2009). Genetic polymorphism analysis of five Vietnam native Ac, Choi, Ho, H’mong and Tre chicken breed using microsatellite. Vietnam J. Biotech., 7(4): 443-53. Le Thi Thu Ha, Nguyen Thi Le Hang, Le Thi Thanh Tam, Nguyen Thi Dieu Thuy and Nguyen Thi Thu (2015). Effect of PIT1 gene polymorphism on performance traits of Tau Vang chicken. JVN Agr. Sci. Tech., 2(56): 114-20. 21. Nguyen Huu Tinh, Nguyen Van Hop, Tran Van Hao, Pham Ngoc Trung and Nguyen Thi Lan Anh (2020a). Production of sire line TS3 selected by EBV and H-FABP, MC4R and PIT-1 genotypes. JAHST, 259: 2-7. 22. Nguyen Huu Tinh, Nguyen Van Hop, Pham Ngoc Trung, Tran Van Hao and Nguyen Thi Lan Anh (2020b). Reproduction of SS1 and SS2 dam lines and parental crossbred sows selected by estimated breeding values and FSHB, PRLR genotype. JAHST, 259: 7-13. 23. Nguyen Ngoc Tuan and Tran Thi Dan (2005). Effect of Halothan gene, Estrogen receptor gene on reproductive performance and meat quality. J. Agr. Sci. Tech., 2&3: 216-31. 24. Nguyen Thi Kim Khang (2006). Genetic factors affecting the omega-3 and omega-6 fatty acid variation in egg yolk. PhD thesis, Uni. of Bonn, Germany. 25. Nguyen Thi Kim Khang and Nguyen Trong Ngu (2013). Effects of Myogenic Factor 5 (MYF5) gene on carcass and meat quality of Mong Cai pigs. The Thai J. Vet. Med., 43(2): 213-18.